Why Us
What Makes SteriLogix Unique
For over a decade, SteriLogix has been helping manufacturers determine how many times their reusable medical devices can be cleaned and sterilized before they are no longer usable. We provide the robust science you need to verify the useful life of your medical device without overburdening your development process.
Our Focus
Our Mission
Our Vision
TeDan Surgical Innovations
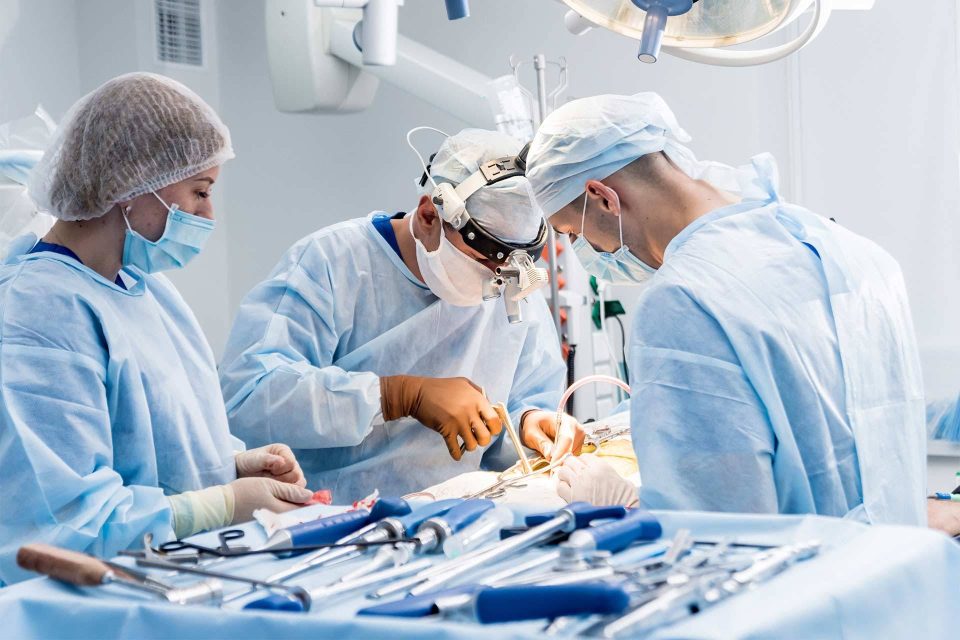
Prioritizing Patient Safety
For a number of practical and economic reasons, many instruments need to be reused from one surgery to the next. With each surgery, there is the potential for an instrument to degrade in terms of function and/or safety. These devices can see more than 500 reprocessing cycles during their useful life!
We believe that these instruments should be just as safe and effective for the very last patient as they are for the first.
The “why” driving SteriLogix is patient safety. We want to make it affordable and timely for manufacturers to conduct the testing needed to ensure that their products are safe and effective for every single patient.

Prioritizing Efficiency: High-Cycle Reprocessing
Other labs conduct numerous types of tests including, cleaning validations, sterilization validations, biocompatibility testing, etc. These are low-cycle tests. As a result, these labs aren’t able to perform the high-cycle tests as quickly or as cost-effectively as SteriLogix can.
SteriLogix is one of the few testing labs that focuses solely on high-cycle reprocessing tests. This makes us less of a one-stop shop, but more responsive to our customers. While an all-inclusive lab can seem attractive, it’s not always the best option when trying to meet a project budget or lead time target.
Let us do the high-cycle testing and then send the devices directly to your choice of a third-party lab for the low-cycle testing.
Adherence To Changing Industry Standards
Testing requirements for reusable medical devices have changed dramatically in recent years due to FDA guidance and EU-MDR requirements.
Manufacturers must now determine the useful life of their reusable instruments and inform the user of this limit, based on testing data. Additionally, per ISO 10993-1:2018, reusable instruments must be preconditioned prior to biosafety testing.
Our specialized equipment and highly-trained staff are able to reprocess medical devices in the same manner that they are reprocessed in a hospital or surgery center sterile products department (SPD).
Helping Manufacturers Manage Their Workload
Useful life and preconditioning testing takes time and needs to be done in a way that accurately replicates reprocessing conditions in the hospital. Some manufacturers have an in-house lab with this type of equipment to do the work themselves.
However, many do not have this equipment and need to contract an outside source, which is why we exist. Some of our clients have in-house equipment but can get overloaded with work so they contract with us to help them manage their workload.
Mazor Robotics (Medtronic)