How SteriLogix Works
Our Services
SteriLogix delivers a complete package of steps and services. By using Standardized Clinical Reprocessing Cycles, your products and materials are brought to market more efficiently and you experience a higher level of confidence in the end result.
Our Services

Receiving Inspection
- Study items are unpacked and an electronic router of all job steps is created in alignment with your IFU and/or testing protocol.
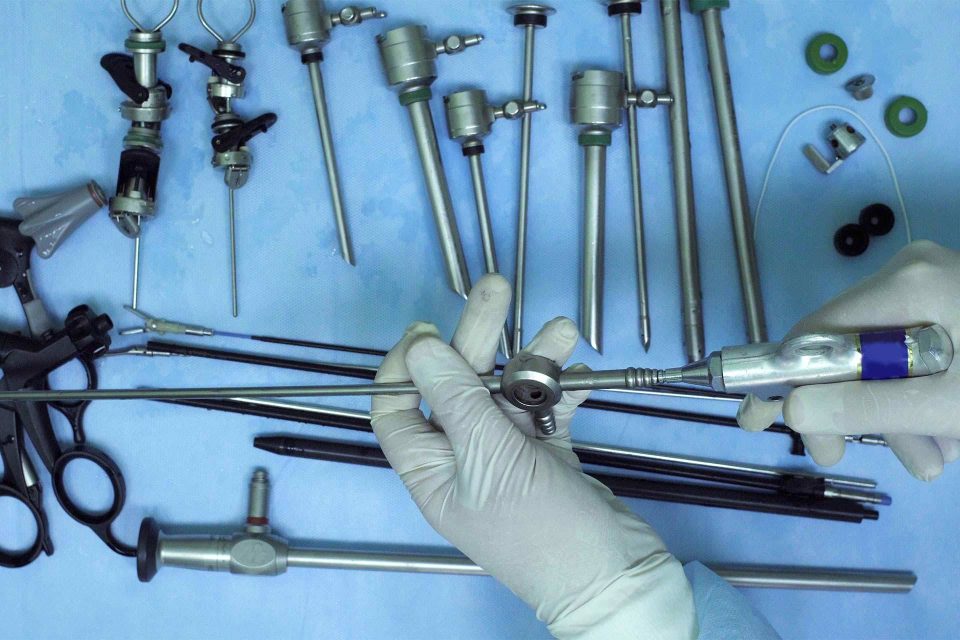
- The test devices are inventoried and photo-documented with specific attention to any regions of interest identified by our clients.
- Time zero (T=0) pictures are always captured prior to the start of testing to document the initial condition of the devices.
Functional Cycles
For complex or multi-component instrumentation, functionality checks can be performed and assessed at interim points throughout testing, when requested by the client. Functional cycles simulate the use of the instrument in the field. Assessments could include any or all of the following activities:
- Soiling the device with bovine blood or other representative soil
- Actuation of moving parts, assembly of mating components, locking/unlocking mechanisms
- QR code scanning, calibrations, powering the device on/off, leak checks, etc.
- Mechanical testing such as compression, tension, torsion, or impact testing
Throughout functionality testing, any changes to the device are evaluated and documented. Typical acceptance criteria for functionality testing is if the device continues to function as intended at each checkpoint.

Manual/Ultrasonic Cleaning Cycles
The manual and ultrasonic cleaning cycle process allows the detergent to access blind holes, cracks, and recesses where debris and bacteria can hide. A detergent and elevated temperatures of 40º – 65ºC are typically used in clinical settings.
- Devices are cleaned manually in an enzymatic or alkaline detergent bath.
- An ultrasonic bath uses high-frequency sound waves to agitate the detergent.

Automated Wash/Disinfect Cycles
After the device has completed the functional cycle and manual cleaning, it can be subjected to an automated wash and/or disinfection cycle.
We utilize the same washer/disinfector equipment found in hospitals and surgery centers to replicate detergent impingements and high-temperature disinfection conditions that the device will see in real-world use. We follow your Instructions for Use (IFU) to set the methods and parameters for this process.
- If you do not yet have an IFU available, our team can recommend typical sterile products department (SPD) parameters for “like-kind” devices.

Autoclave Cycles
After the device has been washed, it will be placed into an autoclave to be sterilized with steam to kill bacteria that may be resistant to the boiling water and detergents in the prior steps.
- A pre-vacuum autoclave unit is one that evacuates the chamber and pulses a steam charge several times before the timed steam charge is initiated. It then dries the devices under a high (greater than 10 inHg) vacuum.
- Most steam charge cycles are 4-18 minutes long at temperatures above 132ºC and below 137ºC.
Lab Certification & Technical Report
We document any changes to the device throughout the testing with a comprehensive record of photographs at prescribed milestones and when changes are first observed.
Your completed study will include a copy of this photo record along with a full certification of what each device was exposed to during this process, including time, temperatures, chemicals, and cycle counts.
Additionally, SteriLogix can provide a full technical report for submission to governing bodies.
The full technical report will include:
- Executive Summary
- Background Materials
- Methods
- Results and Discussion
- Conclusion
- Relevant Photographs and References
Additional Information
Learn More About Our Services

Life Cycle Testing
Learn More

Regulatory Compliance
Learn More

Standardized CRC
Learn More