Life Cycle Testing
Efficient Testing For Medical Device Reprocessing
In order to meet regulatory expectations and design considerations, a recent trend in medical device reprocessing assessment is to conduct life cycle (or durability) testing.
Medical device life cycle testing repeatedly exposes a medical device to the manufacturer’s prescribed reprocessing procedures (or accepted industry standard procedures) and effectively ages the parts to simulate a lifetime of use.
SteriLogix is proud to be exceedingly efficient at conducting these tests for our clients.

Regulatory Expectations and Design Considerations
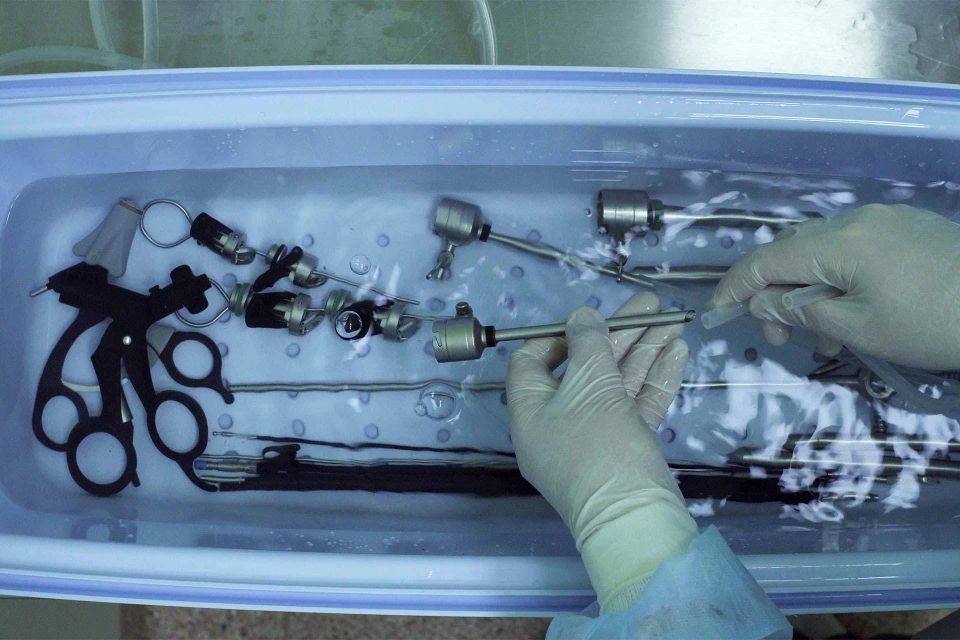
Reusable medical devices must be reprocessed after each surgical use so that they are clean and sterile for the next procedure to ensure the safety of the end user.
The protocol for preparing the device for reuse, following the manufacturer’s IFU, and exposing the device to the same reprocessing conditions the device will see in a hospital or surgery center SPD, is referred to as a Clinical Reprocessing Cycle (CRC).
The cleaning agents and processes associated with CRC washes and steam sterilization can degrade the markings, features, finish, form, function, and other characteristics of a reusable medical device over time.
CRC testing allows manufacturers to collect data that reflects the real-world performance of their devices for the following purposes:

-
Useful Life Assessment
Understanding the CRC performance of the device or instrument can help determine the product’s useful life and when it should be removed from service.
To comply with current ISO, FDA, and EU MDR regulations, manufacturers of reusable instruments, trays, surgical kits, and similar products must perform CRC validations on final products.
They must also evaluate biological safety, cleanability, and sterilization requirements at the maximum number of validated reprocessing cycles.
-
New Design & New Materials Assessment
Understanding CRC performance can also be useful in device design and development. Current device designs are often more complex than legacy designs, which can make the effects of cleaning, disinfecting, and sterilizing a question mark.
Additionally, new materials and manufacturing processes must be evaluated to assess the impact of reprocessing on performance.
-
Field Issue or Corrective Action Assessment
CRC testing can help manufacturers replicate a reprocessing-related field issue and determine the source of the problem. The same testing can then be repeated to verify that the corrective actions taken have properly addressed the reprocessing issue.
-
Preconditioning for Biosafety Validations
It is important to ensure that a reusable device is safe and effective for the very last patient.
Validations of biological safety, cleanability, and sterilization should all be conducted after exposing the device to the maximum number of validated reprocessing cycles.
CRC testing can precondition “or age” the device in an accelerated manner so that they are suitable for these biosafety tests.
CRC Life Cycle Testing

In order to meet these regulatory expectations and design considerations, it is essential to conduct medical device life cycle tests. Life cycle tests, sometimes referred to as durability tests, involve estimating the number of uses that a device will experience over its expected lifetime and then subjecting it to that number of CRCs.
A CRC life cycle test repeatedly exposes the device to the manufacturer’s prescribed reprocessing procedures (or accepted industry standard procedures) using the same equipment used by hospitals and surgery centers. This testing effectively ages the parts to simulate a lifetime of use.
A CRC life cycle test compresses a lifetime of reprocessing into a few weeks or months. Dimensional, functional, and/or mechanical testing may be part of the life cycle test protocol to assess any degradation to these aspects of performance with CRC exposure.
Successful completion of life cycle testing serves to validate the manufacturer’s reprocessing instructions for the device.
Our Process
Testing requirements for reusable medical devices have changed dramatically in recent years. Manufacturers must now determine the useful life of their reusable instruments and inform the user of this limit, based on testing data. Additionally, reusable instruments must be preconditioned prior to biosafety testing. Our focus on only high-cycle reprocessing allows you to collect this data on time and on budget.

Planning
Based on your instructions for use (IFU) and protocols, SteriLogix will create a customized document (or router) outlining the reprocessing steps we will utilize to achieve your testing goals.

Testing
The number of testing cycles will be set based on your study’s objectives. This will be used to validate the useful life of the device or precondition it for other post-exposure validation testing.

Documentation
Upon completion, you will receive a test certification or technical report documenting the reprocessing steps and number of reprocessing cycles.
Implant Direct
Additional Information
Learn More About Steps & Services

Regulatory Compliance
Learn More

Standardized CRC
Learn More
Steps & Services
Learn More