Instrument Testing and Validation Session
Autoclave Cycles
Posted by: SteriLogix
6 years ago
Summary:
- What is Clinical Re-processing
- Why is it important
- How does it affect us
- How do we respond
- What is reusable device life-cycle testing

What’s New in the 2015 Final Guidance(vs the 1996 Guidance)
Expanded to include information pertaining to validation of reprocessing methods and instructions
- Specific emphasis on importance of proper cleaning & cleaning validation, IMPORTANCE OF WORST-CASE TESTING, importance of device designs that are less challenging to reprocess
- Human factors considerations when validating reprocessing methods and instructions
- Provides greater clarity on documentation to be provided in the different premarket submissions510(k), PMA, de novo, HDE, IDE

The FDA is becoming increasingly concerned about the longevity and efficacy of reusable devices – aside from just sterility. They want a guarantee that the device is safe to use on the 300th patient, not just the 1st.

The following is an excerpt from the guidance document,
Reprocessing Medical Devices in Health Care Settings: Validation Methods and Labeling Guidance for Industry and Food and Drug Administration Staff Document issued on: March 17, 2015 Appendix E of this guidance was updated on June 9, 2017, top of page 20,
https://www.fda.gov/downloads/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm253010.pdf
” L. Reuse Life 20 The labeling should either 1) inform the user how many times the device can be reused, based on testing; or 2) provide the user with a mechanism or method to ascertain whether the device has exceeded its use life.
……………………..labeling that identifies a performance test that should be passed prior to reuse; § labeling that recommends visual inspection along with acceptance or failure criteria (e.g., unacceptable deterioration such as corrosion, discoloration, pitting, cracked seals). ……………………….Reuse life may also be addressed by validating the number of times the product can be reprocessed and reused, and providing this specification in the labeling. If the reuse life of a device is limited to a specific number of use/reprocessing cycles, the labeling should also describe a specific tracking method for the number of reuse cycles. “
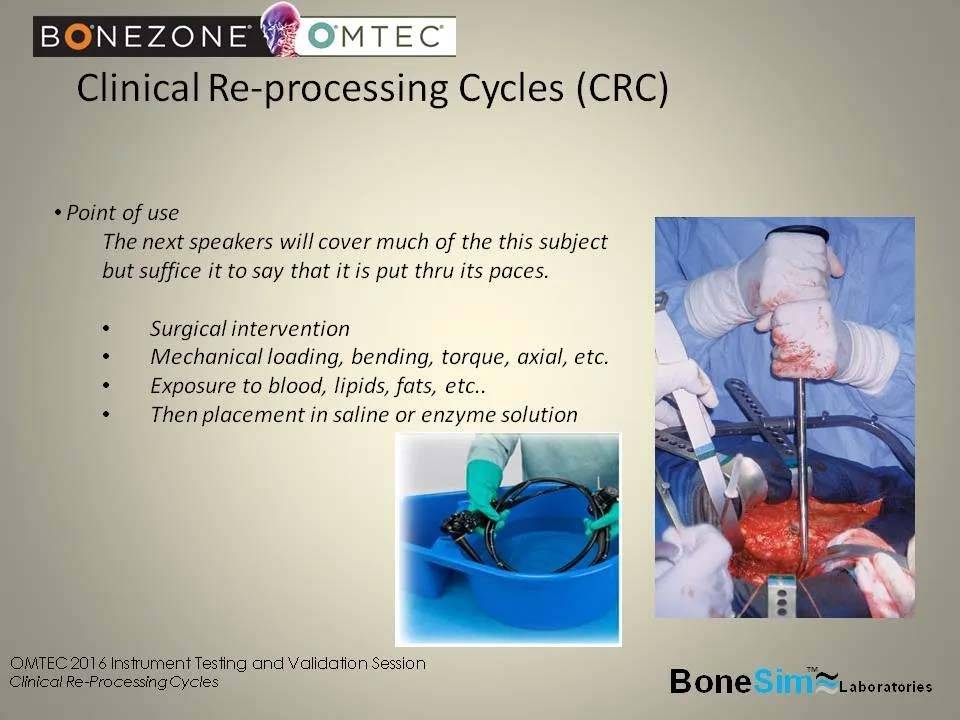
Point of Use
– Surgical intervention
– Mechanical loading, bending, torque, acial, etc.
– Exposure to blood, lipids, fats, etc.
– Then placed in saline or enzyme solution
Terminal Sterilization (autoclave, chemical, ETO, etc.)
Focusing on steam sterilization
Hospital SPDs use longest, highest temperature cycles when IFU is unclear, not available or considered to be not to their standard

Cleaning/disinfecting automatic
Rinse, enzymatic soak, detergent wash, rinse, dry

Why it’s important to us:
Processing instructions i.e., etching, material choice, passivation and passive layer compromise

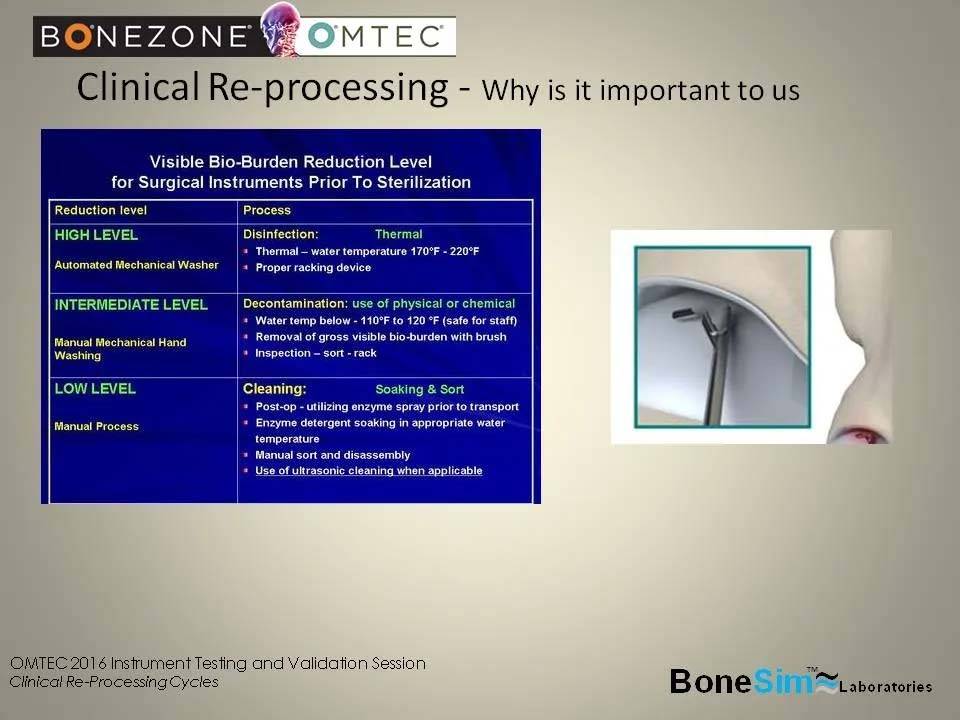
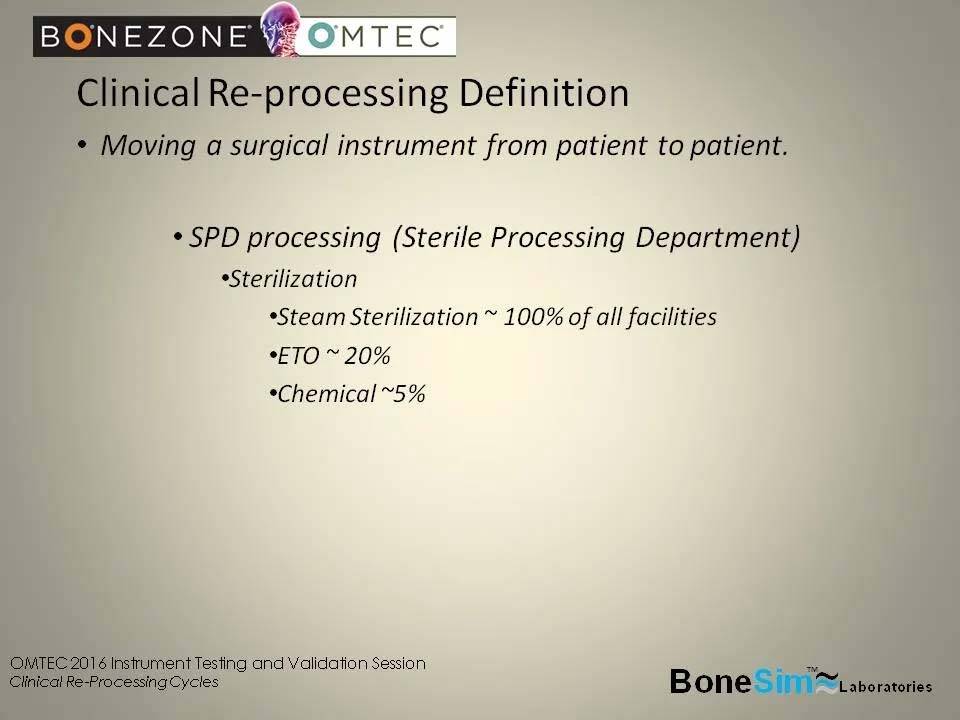
Clinical Re-Processing Definition
-Moving a surgical instrument from patient to patient
– SPD processing (Sterile Processing Department)
– Cleaning/disinfecting
– Manual or Automatic
– Manual: washing with brush in solution
– Automatic: Ultrasonic or Automated Washer
Most facilities use a combination of manual cleaning and automatic cleaning

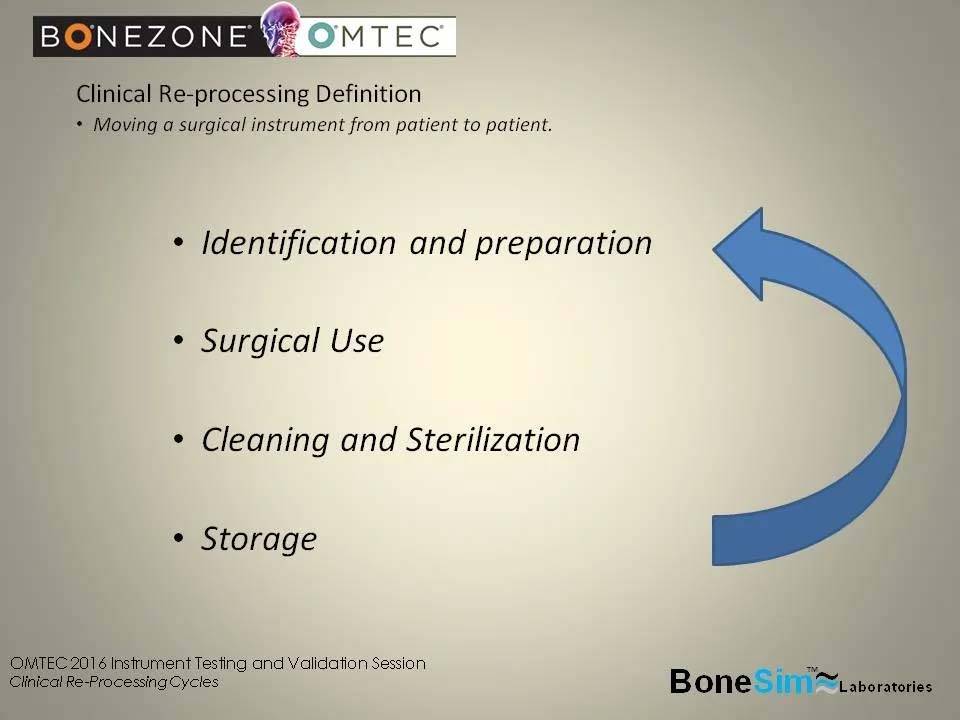
– Identification and preparation
– Surgical use
– Cleaning & Sterilization
– Storage
Choose worst case known facility parameters
-Pros:
Creates data set at worst case conditions (maybe)
Minimizes risk of failures from facilities over processing
Allows standards to be set for CRC
Reduces cost & timeline
– Cons
Failures in testing may not be representative of design
Potential over design
May have subjective findings

SPD (Sterile Processing Department) processing
– Cleaning/disinfecting – manual
Detergent or enzymatic detergent with manual brushing and removal of debris

– Initial soaking
– Usually in the OR at point of use
– Saline or enzyme solution to prevent drying and coagulation to enable efficient cleaning

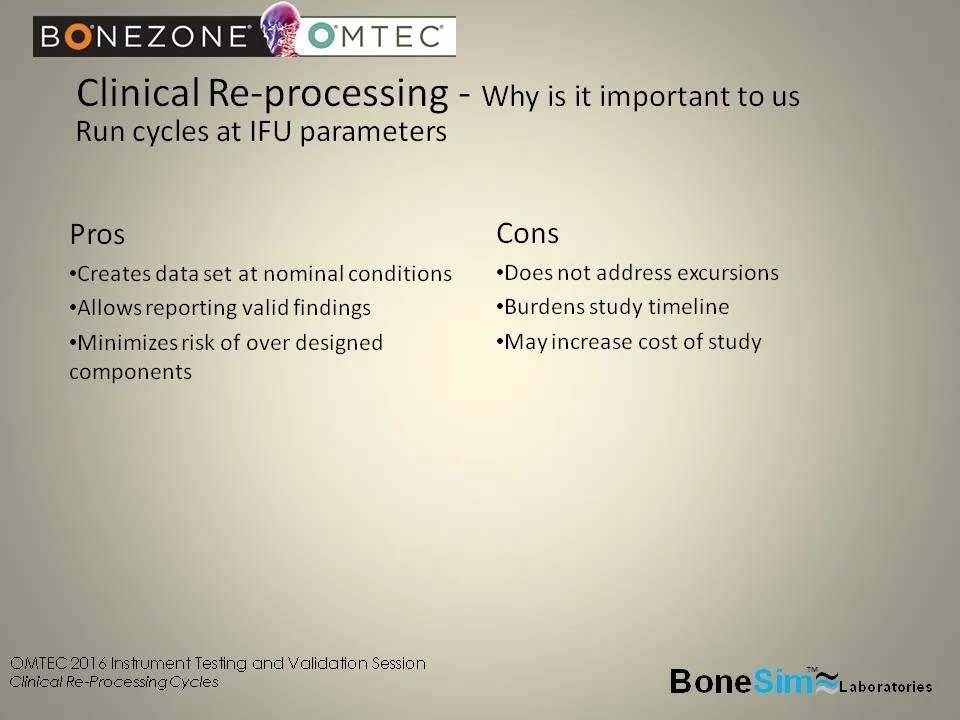
Why it’s important to us
– Run cycles at IFU parameters
Pros:
Creates data set at normal conditions
Allows reporting valid findings
Minimizes risk of over designated components
Cons:
Does not address excursions
Burdens study timeline
May increase cost of study
SPD (Sterile Processing Department) processing
– Sterilization
– Steam Sterilization ~ 100% of all facilities
– ETO ~ 20% of all facilities
– Chemical ~ 5% of all facilities
What are the excursions and parameters to design to:
Two choices
- Run CRC studies at IFU parameters
- Run CRC studies at worst case known facility parameters

The FDA cars about the longevity of efficacy and sterility guarantee

It’s important for material selection

What are the excursions and parameters to design to:
- Time
- Temperature
- Pressure
- Exposure

- Identification and preparation
- Surgical Use
- Cleaning and Sterilization
- Storage
Drilling down…start at the beginning of a case…….
- Instruments are Inspected, Functionally checked and placed on OR/Mayo stand
- Point of use in the OR
- OR soak, sterile distilled water only
- In/out of OR Sprayed enzymatic/foam (breakdown proteins, prevent drying)
- SPD processing (Sterile Processing Department)
- Initial cleaning (enzyme/detergent), flush, brush, air…
- Cleaning/disinfecting (ultrasonic, automated washer)
- Sterilization
Clinical Re-processing Cycles (CRC)
- Instruments are Identified, Inspected, Functionally checked and placed on OR/Mayo stand
- FDA calls this the Point Of Use Processing
Categories:
Ready to Verify the Life Cycle of Your Reusable Medical Devices?
Utilizing our Clinical Reprocessing Cycles (CRC) testing provides a technical, non biased, certified validation so that you can be confident your materials and products are able to withstand the rigors of reprocessing.


