Durability Testing of UDI Labeling
Durability Testing
Posted by: Jack Jennings
1 year ago
Introduction
The world of medical devices is constantly evolving, with new advancements and technologies being introduced to improve patient care. One such advancement is the implementation of Unique Device Identification (UDI) on reusable medical devices. These unique codes are assigned to each medical device, allowing for better tracking, traceability, and overall patient safety.
To ensure the accuracy and reliability of UDI labeling, rigorous testing requirements have been put in place. This testing is essential to ensure that the labels remain intact and legible, even after repeated use and cleaning. Accurate and durable UDI labeling is of utmost importance as it directly impacts patient safety and regulatory compliance.
Complying with UDI requirements is a crucial step for manufacturers and labelers of medical devices. It involves understanding the regulations set forth by regulatory bodies such as the FDA and ensuring that the necessary steps are taken to meet those requirements.
Accurate and complete data submission is essential to ensure proper UDI implementation. It enables healthcare professionals to access relevant information about the device, track its usage, and address any safety concerns effectively.
By complying with UDI requirements and submitting accurate data to GUDID, manufacturers and labelers contribute to enhancing patient safety, facilitating medical device tracking, and improving post-market surveillance efforts.

Durability Testing of UDI Labeling
Durability testing plays a crucial role in ensuring the effectiveness and longevity of UDI labeling on reusable medical devices. As these devices are subjected to rigorous use and frequent sterilization, it is imperative that the UDI labels remain intact and legible throughout their lifecycle.
Durability testing is essential for UDI labeling on reusable medical devices due to the following reasons:
- Patient Safety: Accurate and durable UDI labeling is vital for patient safety as it enables proper identification and tracking of medical devices. A worn-out or illegible label can lead to confusion, misidentification, and potential harm to patients.
- Regulatory Compliance: Regulatory bodies such as the FDA require durable UDI labeling as part of their guidelines. Compliance with these requirements ensures that manufacturers meet the necessary standards for device traceability and safety. Durability testing in the medical device industry comes with its own set of challenges and requirements:
• Harsh Environments: Reusable medical devices are exposed to various harsh environments during their lifecycle, including high temperatures, moisture, chemicals, and physical stress. Durability testing must replicate these conditions to assess the performance of UDI labeling under realistic scenarios.
• Material Compatibility: Different types of UDI labels may have varying levels of compatibility with different device materials. It is crucial to test the durability of UDI labels on a range of materials commonly used in medical devices, such as stainless steel, plastics, or ceramics.
Conducting thorough durability testing for UDI labeling offers several benefits:
- Reliable Traceability: Durable UDI labels ensure accurate traceability throughout the entire lifespan of a reusable medical device. This helps healthcare professionals track devices, monitor their usage history, identify recalls or adverse events, and ensure proper maintenance.
- Cost Savings: By identifying and addressing potential labeling issues early on through durability testing, manufacturers can avoid costly rework or product recalls. Robust UDI labels reduce the risk of misidentification and associated financial implications. Several methods are commonly used to evaluate the durability of UDI labeling:
- Abrasion Testing: This method assesses the label’s resistance to wear and tear caused by repeated contact with surfaces or abrasive substances.
- Chemical Resistance Testing: Chemical resistance testing determines a label’s ability to withstand exposure to various chemicals used in medical device cleaning or sterilization processes.
- Temperature Cycling Testing: This method subjects UDI labels to extreme temperature changes to evaluate their performance under different environmental conditions.
- UV Exposure Testing: UV exposure testing simulates prolonged exposure to sunlight or artificial UV radiation, assessing the label’s resistance to fading or degradation.
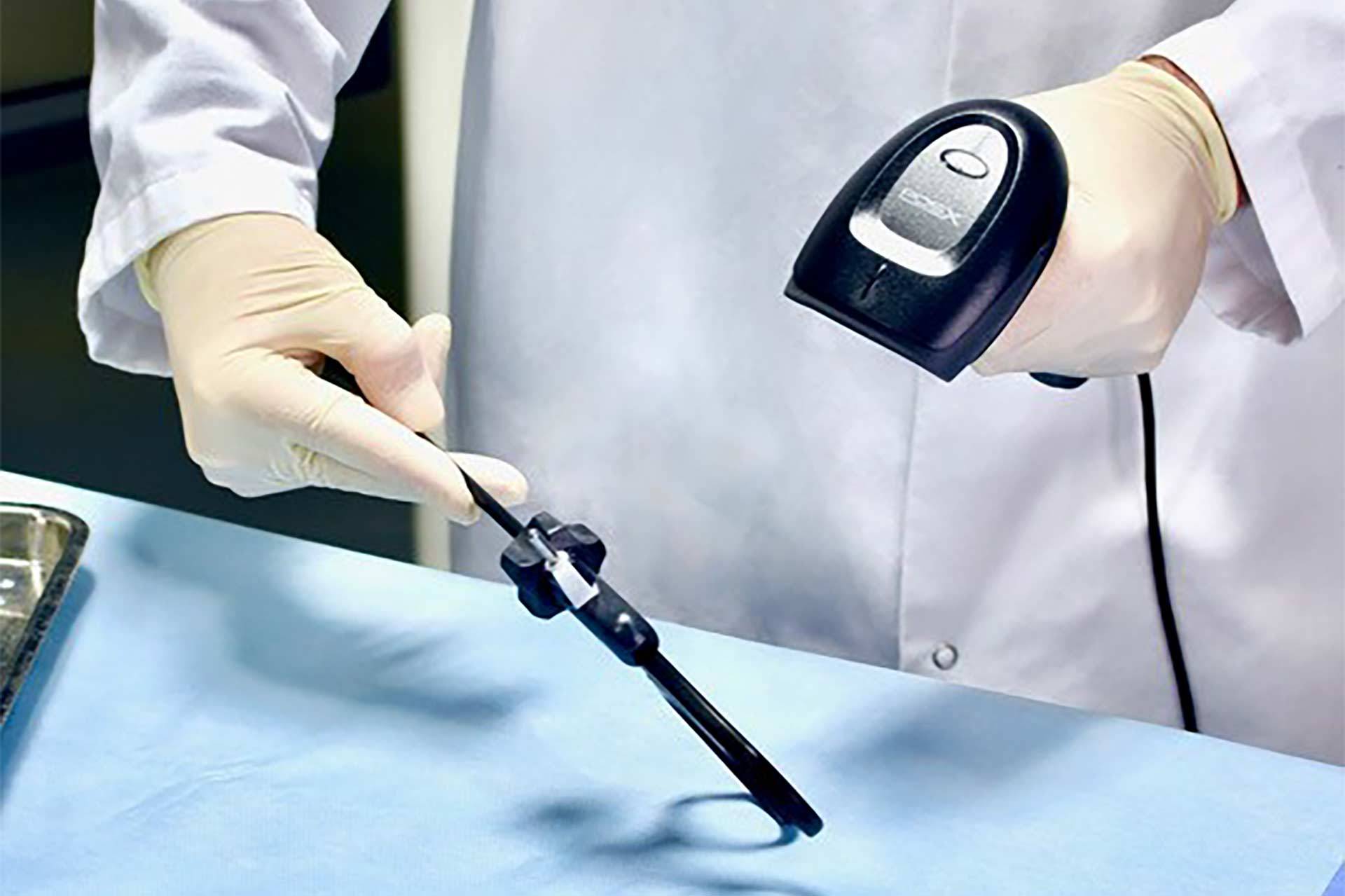
Durability testing is a critical aspect of UDI labeling for reusable medical devices. By ensuring the longevity and legibility of UDI labels, manufacturers can enhance patient safety, comply with regulations, and enable accurate device traceability throughout its lifecycle.
Reprocessing and sterilization are crucial steps in the lifecycle of reusable medical devices. However, these processes can have a significant impact on the durability of UDI labeling. Here are some key considerations when it comes to reprocessing and sterilization:
• Impact on UDI labeling durability: Reprocessing and sterilization procedures can subject medical devices to harsh conditions, including high temperatures, chemicals, and physical stress. These conditions may compromise the integrity of UDI labels, leading to fading, smudging, or complete loss of the labeling information.
- Importance of proper cleaning procedures: Proper cleaning procedures play a vital role in maintaining the integrity of UDI labels. Thorough cleaning removes any potential contaminants that could interfere with the legibility and durability of the labels. It is essential to follow manufacturer-recommended cleaning protocols to ensure optimal label performance.
- Challenges and considerations: Sterilization methods such as steam autoclaving, ethylene oxide (EtO) sterilization, or gamma irradiation can pose challenges for UDI labels. Some labeling materials may not withstand these processes, causing degradation or illegibility. Additionally, the presence of moisture during sterilization can further impact label durability.
To overcome these challenges, device manufacturers should consider selecting UDI labeling materials that are specifically designed to withstand reprocessing and sterilization methods commonly used in healthcare settings. Conducting thorough testing to evaluate label performance under these conditions is essential for ensuring accurate and durable identification throughout the device’s lifecycle.
By addressing reprocessing and sterilization considerations in UDI labeling, manufacturers can enhance patient safety by ensuring clear and legible identification even after multiple uses and sterilization cycles.
Conclusion
To wrap up, we have provided a brief introduction to the world of UDI labeling on reusable medical devices and the importance of durability testing. Here are the key takeaways:
- UDI labeling plays a crucial role in ensuring patient safety and effective device management.
- Durability testing is essential to ensure that UDI labels remain intact and readable throughout the device’s lifespan.
In conclusion, accurate and durable UDI labeling is vital for compliance with regulations and patient safety. Manufacturers should prioritize the implementation of robust durability testing methods to ensure the longevity and effectiveness of UDI labels on reusable medical devices.
At SteriLogix, our sole focus is conducting high-cycle durability tests that reflect real-world reprocessing conditions. We specialize in helping OEMs validate the longevity and legibility of their UDI labeling.
Ready to Verify the Life Cycle of Your Reusable Medical Devices?
Utilizing our Clinical Reprocessing Cycles (CRC) testing provides a technical, non biased, certified validation so that you can be confident your materials and products are able to withstand the rigors of reprocessing.

