Client FAQ – What is Block Cycling?
Client FAQs
Posted by: Jack Jennings
11 months ago
Reprocessing durability studies are typically very time-consuming. This is because one reprocessing cycle usually takes 3-5 hours from start to finish and involves the following operations or job steps:
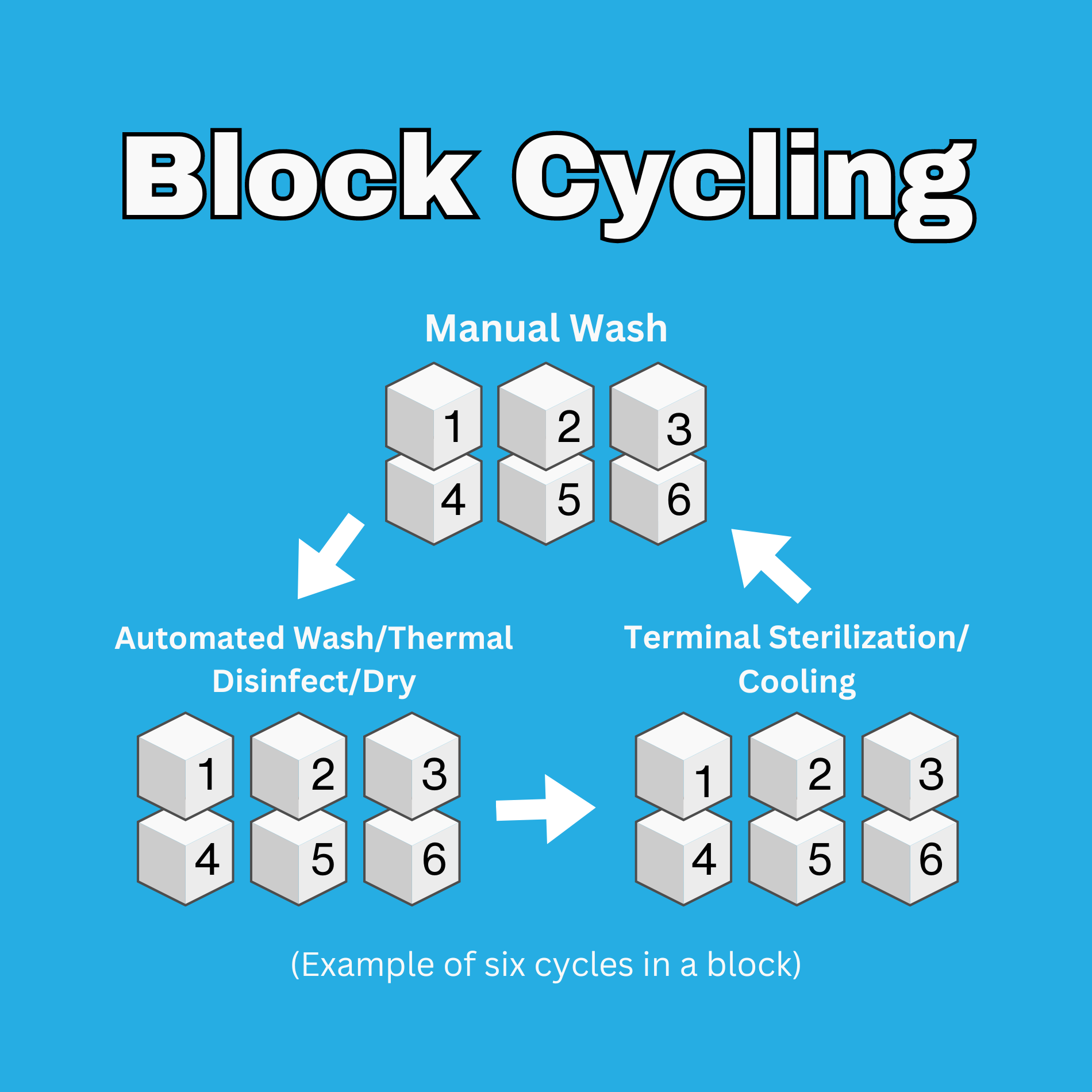
- Manual Wash (20-45 minutes)
- Automated Wash and Thermal Disinfect (60-90 minutes with dry cycle)
- Terminal Sterilization (90-120 minutes with cooling to room temperature)
This reprocessing cycle must then be repeated multiple times. For most studies, replicating a lifetime of use requires running 100 to 500 reprocessing cycles, or more. Additionally, these studies often involve a large number of samples or large devices (such as full-size trays). These larger studies may necessitate multiple iterations of one, or all, of the job steps above to process the full set of devices. As you can see, study time can add up quickly.
We are often asked the following question: Is there is any way to speed up the process? Unfortunately, there is very little that can be done to speed up the individual job steps without compromising the validity of the data obtained. However, in many cases, the overall length of the study can be shortened by manipulating the order of the job steps. With proper assessment of the study parameters (operations, materials, objectives, etc.) we can often run the job steps in small groups of repetitive blocks, rather than sequential order, without compromising the validity of the data obtained. We call this “block cycling”. We review this option with the client and proceed with their approval.
METHODOLOGY + APPLICATION
In a surgical setting, devices must be reprocessed in a specific order since they are soiled during the surgical procedure. Therefore, the reprocessing protocol in the instructions for use (IFU) is written with the expectation that the soiled parts will be cleaned, then disinfected, and then sterilized. After this, they are returned to surgery, soiled again, and the process must be repeated in a serial manner. This is not the case in a laboratory study where the devices will not be soiled, and where the sole purpose of the testing is to expose the devices to the cumulative effects of the temperatures, pressures, impingements, and chemistries of the reprocessing cycle itself.
Life cycle conditioning, as part of a non-clinical laboratory study, is a function of cumulative exposure to the expected cleaning and sterilization processes. For most products, the order of the reprocessing operations does not impact the cumulative exposure effects. Whether a part is washed then autoclaved, or autoclaved then washed, it sees the same operational exposure. While the order of the operations generally has no effect, it is important to fully complete all the sub-steps within a single operation completely and in the order specified. This is because the operation may induce thermal or pressure hold times and/or gradients that, if altered, can have an impact on reprocessing effects. For example, increasing the dwell time at peak temperature in the autoclave, or shortening the cool time of the autoclave cycle. Likewise, rinses intended to wash away detergents need to come after the detergent wash step in a cleaning operation. Block cycling does not involve any change to the sub-steps within an operation itself. Additionally, block cycling does not alter or disrupt the transition from one operation to the next. Each operation is run to completion before re-running that same operation or moving on to a different operation.
The benefit of block cycling is that it allows the testing to be accelerated in a way that does not compromise the integrity of the test data. When conducting reprocessing studies, machine availability is one of the primary rate limiters. Having the ability to run additional cycles on a machine while it is available, or while the equipment for the next operation is occupied, allows the testing to continue rather than sitting idle. It is for this same reason that excessive block cycling is not useful. Excessive cycling of the same operation would occupy that machine/equipment for an extended amount of time and slow the pace of testing. In practicality, blocks limited to no more than 10 operations typically provide optimal efficiency. When applied in this manner, block cycling can reduce testing time up to 25%. This can be substantial when you consider that block cycling could shorten a 6-week study to 4 weeks or a 24-week study to 18 weeks.
CONSIDERATIONS FOR USE
As I mentioned above, it’s important to assess all aspects of a study to determine if block cycling can be applied with minimal risk of compromising the validity of the data obtained. Here are some of the common considerations in determining if block cycling can be applied to your study:
- The study does not require soiling the devices.
- The devices involved are fabricated from metallic materials commonly used in medical devices.
- The devices involved are fabricated from polymeric materials commonly used in medical devices and there is no reasonable expectation of sensitivity to the order of the operations.
- The protocol involves standard cleaning, disinfection and sterilize cycles with no aspect that would necessitate running the operations in a sequential manner.
- The objective of the study is to expose the devices to the cumulative effects of the temperatures, pressures, impingements, and chemistries that occur with repeated reprocessing. This can be for the purpose of assessing the useful life of the device, or to precondition the device to the end of its anticipated useful life.
If you have further questions about block cycling or would like to set up a consultation on a specific reprocessing study, please contact us and our team of engineers will be happy to assist.
Ready to Verify the Life Cycle of Your Reusable Medical Device?
Utilizing our Clinical Reprocessing Cycles (CRC) testing provides a technical, non biased, certified validation so that you can be confident your materials and products are able to withstand the rigors of reprocessing.

